FDA Panel Backs More Restricted Hydrocodone Prescribing
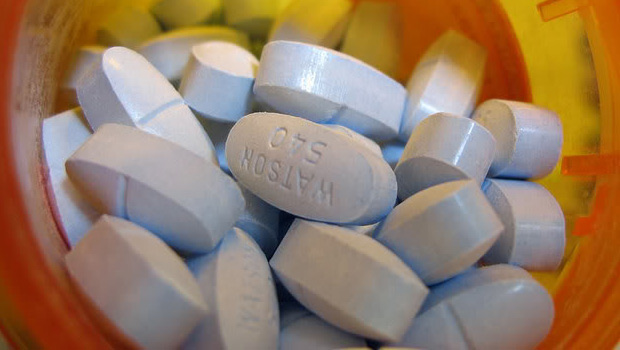
Vicodin, Norco, and other hydrocodone combination products should be reclassified as schedule II drugs, because stricter controls on prescribing are needed to address the current epidemic of abuse, misuse, and diversion of these products in the United States, the majority of a Food and Drug Administration advisory panel has recommended.
At the end of a 2-day meeting on Jan. 25, the FDA’s Drug Safety and Risk Management Advisory Committee voted 19 to 10 that these products should be rescheduled from schedule III under the Controlled Substances Act (CSA), to schedule II. Schedule III allows written or oral prescribing, and 5 refills within 6 months, while schedule II drugs require handwritten prescriptions and other restrictions. The meeting was held in response to a request from the US Drug Enforcement Administration that these products be rescheduled because of evidence they are overprescribed, diverted, and abused.
Panelists said that based on the epidemiologic and pharmacologic data presented during the meeting—which they acknowledged had limitations and was somewhat confusing—the abuse potential and pharmacology of hydrocodone combination products were similar to those of oxycodone and other schedule II drugs.
Those voting in favor of rescheduling said that it would result in a net public health benefit by making these drugs less available for abuse and reducing the amount that ends up in medication cabinets and on the street. And they acknowledged that there is little concrete evidence that tighter regulation would reduce the widespread abuse, misuse, and diversion of these products. In addition, rescheduling is likely to have some negative consequences, panelists noted.
But panelists voting against rescheduling said there was no evidence that rescheduling would have an impact on abuse and diversion, and would likely result in an increase in the use of heroin and other illicit drugs, and problems with other schedule III drugs. They were also concerned about the negative consequences of rescheduling on the treatment of pain, with the increased burden and reduced access to effective pain medications for patients in pain, particularly those in rural areas or those who have difficulty getting to a doctor for a prescription renewal.
Almost all (99%) of hydrocodone used in the world is in the United States, where hydrocodone combination products are the most widely prescribed products: In 2011, there were about 131 million prescriptions for the combination hydrocodone analgesic products, for 47 million people, compared with 34.6 million for oxycodone combination drugs prescribed for 15.1 million patients, according to national prescription data cited by the FDA. About 40% of the hydrocodone combination analgesics are prescribed by primary care practitioners.
The FDA’s analysis of the national data indicates that hydrocodone products are widely abused. High school students are more likely to abuse hydrocodone products than oxycodone products, because there are so many more hydrocodone prescriptions and wider availability of leftover drugs in homes. However, hydrocodone combination products have lower abuse ratios than oxycodone combination products, according to the FDA reviewers, who acknowledged that calculating accurate abuse ratios is complicated.
Panelists strongly recommended close study of the impact of the scheduling change on prescribing practices, abuse, and diversion. There should be a transition period in the rescheduling process so patients with appropriate needs for the drug and prescribers can adjust to the tighter regulations and avoid losing access to the drug abruptly.
Full article by Elizabeth Mechcatie, Rheumatology News Digital Network